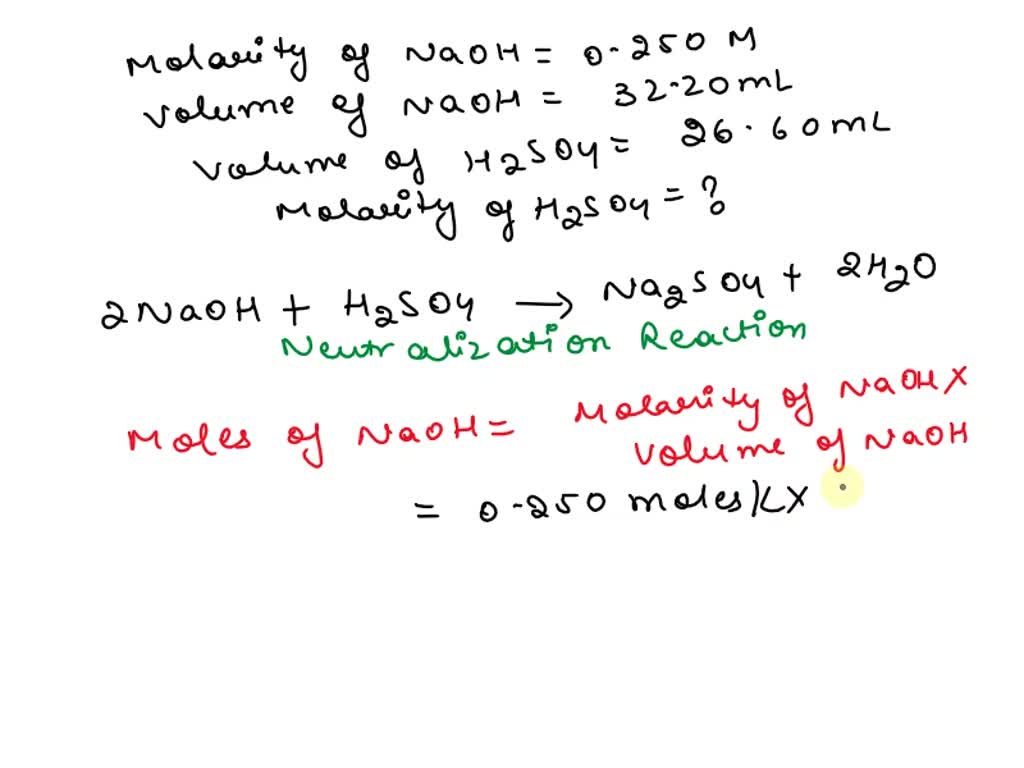H2So4 Titration With Naoh Calculation . Average the values for the total volumes of naoh added. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. first determine the moles of \(\ce{naoh}\) in the reaction. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: If a third titration was required, average the two.
from www.numerade.com
a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. If a third titration was required, average the two. several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Average the values for the total volumes of naoh added. first determine the moles of \(\ce{naoh}\) in the reaction.
SOLVED Q3 In a titration of sulfuric acid against sodium hydroxide
H2So4 Titration With Naoh Calculation Average the values for the total volumes of naoh added. first determine the moles of \(\ce{naoh}\) in the reaction. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Average the values for the total volumes of naoh added. If a third titration was required, average the two. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that.
From www.coursehero.com
[Solved] Using the following equation 2 NaOH + H2SO4 2 H2O + Na2SO4 H2So4 Titration With Naoh Calculation From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. If a third titration was required, average the two. in this process, 2 moles (the molecular. H2So4 Titration With Naoh Calculation.
From exoqwdiya.blob.core.windows.net
H2So4 Titration Graph at Jewell Hamilton blog H2So4 Titration With Naoh Calculation in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. Average the values for the total volumes of naoh added. From the mole ratio, calculate the moles. H2So4 Titration With Naoh Calculation.
From chemistry291.blogspot.com
H2SO4 + NaOH Sulfuric acid(H2SO4) and Sodium hydroxide(NaOH)What is H2So4 Titration With Naoh Calculation in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. first determine the moles of \(\ce{naoh}\) in the reaction. several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. in a titration of sulfuric acid. H2So4 Titration With Naoh Calculation.
From fity.club
H2so4 Naoh Naoh H2so4 Sulfuric Acidh2so4 And H2So4 Titration With Naoh Calculation a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. If a third titration was required, average the two. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. first determine the moles of \(\ce{naoh}\) in the reaction. Average the values. H2So4 Titration With Naoh Calculation.
From www.youtube.com
Neutralisation Reaction Sulfuric Acid and Sodium Hydroxide Balancing H2So4 Titration With Naoh Calculation From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. Average the values for the total volumes of naoh added. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. If a third titration was required, average the two. first determine the moles of \(\ce{naoh}\) in the. H2So4 Titration With Naoh Calculation.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water H2So4 Titration With Naoh Calculation From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed.. H2So4 Titration With Naoh Calculation.
From www.numerade.com
SOLVED Q3 In a titration of sulfuric acid against sodium hydroxide H2So4 Titration With Naoh Calculation in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: several practice multiple choice and short answer questions at the end of each lesson to. H2So4 Titration With Naoh Calculation.
From byjus.com
64. Equal volumes of molar HCL and H2SO4 are neutralized by dilute NAOH H2So4 Titration With Naoh Calculation If a third titration was required, average the two. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. several practice multiple choice and short. H2So4 Titration With Naoh Calculation.
From cesxafgg.blob.core.windows.net
Titration H2So4 And Naoh at Jessica Ludwig blog H2So4 Titration With Naoh Calculation several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. Average the values for the total volumes of naoh added. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. in a titration of sulfuric acid. H2So4 Titration With Naoh Calculation.
From allisson-blogweber.blogspot.com
H2so4 Naoh Balanced Equation H2So4 Titration With Naoh Calculation first determine the moles of \(\ce{naoh}\) in the reaction. If a third titration was required, average the two. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. From the mole. H2So4 Titration With Naoh Calculation.
From fity.club
H2so4 Naoh Naoh H2so4 Sulfuric Acidh2so4 And H2So4 Titration With Naoh Calculation From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. Average the values for the total volumes of naoh added. If a third titration was required,. H2So4 Titration With Naoh Calculation.
From www.buzzle.com
Titration of Sulfuric Acid and Sodium Hydroxide H2So4 Titration With Naoh Calculation in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: If a third titration was required, average the two. first determine the moles of \(\ce{naoh}\) in the reaction. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. in this process, 2 moles (the molecular weight of a substance expressed. H2So4 Titration With Naoh Calculation.
From www.numerade.com
SOLVED Questions Calculate the molarity of a sodium hydroxide (NaOH H2So4 Titration With Naoh Calculation several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. Average the values for the total volumes of naoh added. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one. From the mole ratio, calculate the moles. H2So4 Titration With Naoh Calculation.
From www.numerade.com
SOLVED a titration was performed on a 25.0 mL sample of H2SO4 using 17 H2So4 Titration With Naoh Calculation in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. first determine the moles of \(\ce{naoh}\) in the reaction. If a third titration was required, average the two. Average the values for the total volumes of. H2So4 Titration With Naoh Calculation.
From fity.club
Naoh H2So4 Titration With Naoh Calculation first determine the moles of \(\ce{naoh}\) in the reaction. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. in this. H2So4 Titration With Naoh Calculation.
From mavink.com
H2so4 Titration Curve H2So4 Titration With Naoh Calculation first determine the moles of \(\ce{naoh}\) in the reaction. Average the values for the total volumes of naoh added. If a third titration was required, average the two. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: From the mole. H2So4 Titration With Naoh Calculation.
From www.slideserve.com
PPT Theoretical Yield Which Reactant is Limiting? PowerPoint H2So4 Titration With Naoh Calculation several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: first determine the moles of \(\ce{naoh}\) in the reaction. Average the values for the total volumes of naoh added. From the mole ratio, calculate the moles. H2So4 Titration With Naoh Calculation.
From www.numerade.com
SOLVED The concentration of magnesium hydroxide in Colgate ^IM H2So4 Titration With Naoh Calculation several practice multiple choice and short answer questions at the end of each lesson to test understanding of the materials. Average the values for the total volumes of naoh added. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. . H2So4 Titration With Naoh Calculation.